Tattoo inks are controversially classified as “hygiene products” in some regulatory systems, despite being injected into the skin and remaining permanently. This tattoo ink safety controversy raises concerns about weak regulation, insufficient testing, and increased tattoo health risks for consumers.
KinInk.co.kr
“Tattoo needles are medical devices—so why are inks treated like toothpicks?”
During South Korea’s 2025 National Assembly audit, serious concerns were raised about the confusing and inadequate regulatory framework governing tattoo inks—sparking renewed debate over tattoo safety.
Did you know that tattoo inks, which are injected directly into the skin and remain in the body for life, are legally managed in the same category as disposable toothpicks and wooden chopsticks—as mere “hygiene products”?
Table of Contents

The Current Reality of Tattoo Inks Downgraded to “Hygiene Products” (2025 Update)
On June 14, 2025, responsibility for regulating tattoo inks was transferred from the Ministry of Environment to the Ministry of Food and Drug Safety (MFDS), raising hopes for stronger safety oversight.
In practice, however, the transition has exposed serious regulatory gaps and systemic weaknesses.
A Split Regulatory System: “Needles as Medical Devices, Inks as Hygiene Products”
- Tattoo needles: Due to their invasive nature, they are under review for regulation at the medical-device level by the Ministry of Health and Welfare.
- Tattoo inks: Regulated by the MFDS under the Hygiene Products Control Act, grouped together with items such as toothpicks and dental floss.
The problem: Given that tattoo inks are injected into the human body and remain there long-term, critics argue that this level of regulation is dangerously inadequate.
Three Major Safety Gaps Highlighted in the 2025 National Audit
- Low market coverage
Only about 10% of businesses were reported to have completed official registration, raising concerns that unregulated products continue to circulate outside the system. - Weak post-market enforcement
Inspections of unregistered businesses were often impossible due to relocation or closure, severely limiting regulatory effectiveness. - Testing and distribution risks
Lawmakers questioned the extremely limited number of sterile and precision inspections, warning that substandard inks could easily reach consumers.
A Critical Enforcement Failure: Only 10% Registration Rate
- As of June 2025, manufacturers and importers of tattoo inks are legally required to register their businesses with the MFDS.
- However, according to audit data released in October 2025:
- Of 105 businesses previously registered with the Ministry of Environment,
- Only 11 companies (9 manufacturers, 2 importers) had completed the new registration—roughly 10%.
- MFDS field investigations found that most unregistered businesses had relocated or shut down, making inspections impossible.
- Even among the five businesses that were inspected, enforcement amounted to little more than basic guidance, with virtually no meaningful follow-up.
Missing Safety Tests: Only One Sterility Inspection
- In 2025, tattoo ink imports dropped by 98% compared to 2022, totaling just 42 import cases.
- While more than 1,000 sterile and precision inspections had been anticipated annually, only one such inspection was actually conducted.
- The remaining 41 imports entered the country in bulk packaging, bypassing import-stage testing under the condition that companies would conduct self-quality inspections within six months—raising serious safety concerns.
The Risks of Substandard Tattoo Inks: Potentially Life-Threatening Effects
According to MFDS findings, defective or contaminated tattoo inks can pose serious risks not only to the skin, but to overall health.
At the core of the issue are failures in controlling:
- Hazardous substances (e.g., heavy metals, preservatives)
- Microbial contamination
- Chemical impurities
Reported adverse effects include:
- Flat warts
- Granulomas
- Uveitis
Severe complications include:
- Mercury poisoning
- Anaphylaxis (life-threatening allergic reactions)
Notably, investigations by the Korea Consumer Agency have detected hazardous substances and regulatory exceedances in commercially available tattoo inks—reinforcing the conclusion that tattoo ink safety cannot be adequately managed under a consumer “hygiene product” framework.
Regardless of Classification, Tattoo Ink Is an Invasive Material
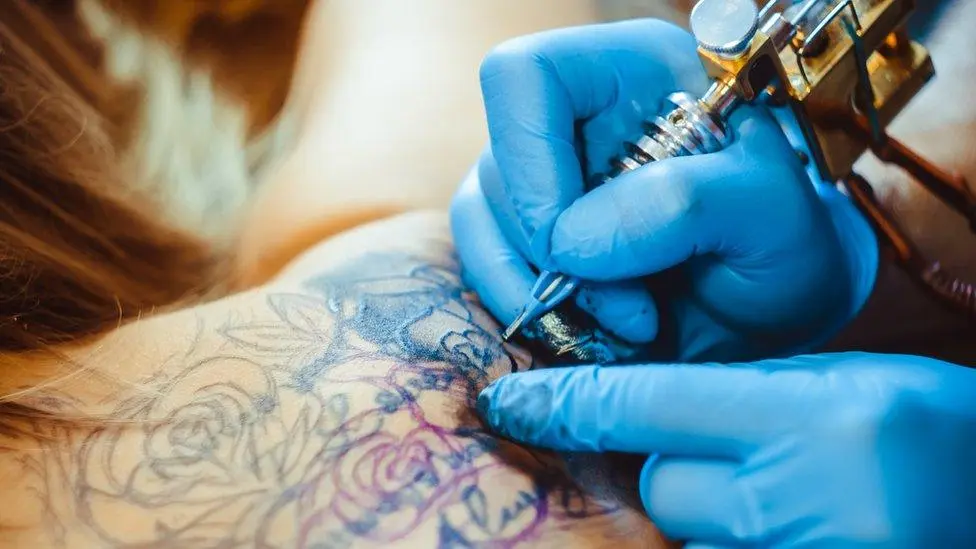
No matter how it is legally categorized, tattoo ink should be regulated based on its risk profile as a substance permanently injected into the human body.
Despite the ambiguity and low regulatory standards currently applied in Korea, Kinink has consistently prioritized skin safety and proactively adopted international-level safety standards.
Kinink goes beyond domestic “hygiene product” requirements by meeting globally recognized benchmarks for safety and ethics.
Kinink’s Safety Commitments
- U.S. FDA Registration
Kinink is officially registered with the U.S. Food and Drug Administration (FDA), demonstrating compliance with stringent safety and manufacturing expectations required in the world’s largest market. - Vegan Certification
Certified vegan, with no animal-derived ingredients and no animal testing—meeting the expectations of customers who value ethical production and ingredient purity. - Sterile Manufacturing & Quality Control
Produced and packaged in sterile environments, with regular internal quality testing to verify freedom from microbial contamination and heavy metals—exceeding minimal self-inspection requirements.
Tattoo inks are not disposable goods. They are invasive materials that remain in the body for life.
Safety standards should reflect that reality.



